ERASMUS+ scholarship for Maike Thal
 In cooperation with the Institute Jean Lamour in Nancy, France, computational investigations on the ternary system Ga-Sn-Pd are done to establish surface models for different surface orientations as well as calculating adsorption energies and reaction paths within the semi-hydrogenation of acetylene at these surfaces. For this our PhD student Maike Thal will study at École des Mines in Nancy for 4 months – funded by the ERASMUS+ student mobility program for higher education. The ERASMUS+ program is thankfully acknowledged for granting financial support. In cooperation with the Institute Jean Lamour in Nancy, France, computational investigations on the ternary system Ga-Sn-Pd are done to establish surface models for different surface orientations as well as calculating adsorption energies and reaction paths within the semi-hydrogenation of acetylene at these surfaces. For this our PhD student Maike Thal will study at École des Mines in Nancy for 4 months – funded by the ERASMUS+ student mobility program for higher education. The ERASMUS+ program is thankfully acknowledged for granting financial support.
|
Cover Art for ACS Catalysis
 Intermetallic compounds–a structurally ordered subclass of multimetallic systems–allow for controlled adjustment of both electronic properties and geometric properties, which can optimize the intermetallic compounds' interaction with reactants. The optimization pathways for intermetallic compound catalysts used in the field of electrochemical energy conversion are explained within the Review. Intermetallic compounds–a structurally ordered subclass of multimetallic systems–allow for controlled adjustment of both electronic properties and geometric properties, which can optimize the intermetallic compounds' interaction with reactants. The optimization pathways for intermetallic compound catalysts used in the field of electrochemical energy conversion are explained within the Review.
|
Review published
 A sustainable energy infrastructure requires chemical energy conversion, i.e. the storage of renewable energy in small chemical molecules and the release of the stored energy upon demand. Electrocatalysis, comprising reactions like hydrogen generation by water splitting, takes a central role in implementing many of the necessary reactions. With their ordered structure, intermetallic compounds provide a unique combination of crystal and electronic structure, thus new (electro)chemical properties. This review comprises nearly 350 publications since 1970 using intermetallic compounds as electrocatalysts in energy-relevant reactions. Special emphasis lies on the so-far applied optimization strategies involving the peculiar properties of the class of intermetallic compounds. Understanding the versatility of intermetallic compounds opens possibilities for further development of heterogeneous catalysts, e.g. electrodes for electrochemical conversions. A sustainable energy infrastructure requires chemical energy conversion, i.e. the storage of renewable energy in small chemical molecules and the release of the stored energy upon demand. Electrocatalysis, comprising reactions like hydrogen generation by water splitting, takes a central role in implementing many of the necessary reactions. With their ordered structure, intermetallic compounds provide a unique combination of crystal and electronic structure, thus new (electro)chemical properties. This review comprises nearly 350 publications since 1970 using intermetallic compounds as electrocatalysts in energy-relevant reactions. Special emphasis lies on the so-far applied optimization strategies involving the peculiar properties of the class of intermetallic compounds. Understanding the versatility of intermetallic compounds opens possibilities for further development of heterogeneous catalysts, e.g. electrodes for electrochemical conversions.
L. Rößner, M. Armbrüster
ACS Catal. 9, 2019, 2018.
doi: 10.1021/acscatal.8b04566
|
Travel Grant for Researcher Exchange
 For the investigation of decapsulating processes in the In-Pd System under methanol steam reforming conditions via atomically resolved TEM microscopy financial support for a Young Scientist exchange to the Ernst-Ruska-centre for microscopy with electrons, Jülich, was granted to our PhD student Nicolas Köwitsch. The European Integrated Centre for the Development of New Metallic Alloys and Compounds (ECMetAC) is thankfully acknowledged for granting financial support. For the investigation of decapsulating processes in the In-Pd System under methanol steam reforming conditions via atomically resolved TEM microscopy financial support for a Young Scientist exchange to the Ernst-Ruska-centre for microscopy with electrons, Jülich, was granted to our PhD student Nicolas Köwitsch. The European Integrated Centre for the Development of New Metallic Alloys and Compounds (ECMetAC) is thankfully acknowledged for granting financial support.
|
 |
Sulfur Spillover on carbon materials and possible impacts on metal-sulfur batteries
 There is currently intense research on sulfur/carbon composite materials as positive electrodes for rechargeable batteries. Preparation of such composites by ball milling or (melt/solution) impregnation are popular approaches to obtain intimate contact between both elements with the hope to improve battery performance. Here, we report that sulfur shows an unexpected “spillover” effect in contact with porous carbons (e.g 1:1 mass ratio) under ambient conditions. When gently mixing sulfur and porous carbon, complete surface coverage takes place within just a few days along with the loss of sulfurs bulk properties (crystallinity, melting point, Raman signals). Sulfur spillover also occurs in presence of a liquid phase. Consequences of this phenomenon are discussed using a sodium-sulfur cell with solid electrolyte membrane. Overall, sulfur spillover on carbon is a so far overlooked phenomenon that we expect to have major relevance for all types of metal-sulfur batteries with porous carbon as support. There is currently intense research on sulfur/carbon composite materials as positive electrodes for rechargeable batteries. Preparation of such composites by ball milling or (melt/solution) impregnation are popular approaches to obtain intimate contact between both elements with the hope to improve battery performance. Here, we report that sulfur shows an unexpected “spillover” effect in contact with porous carbons (e.g 1:1 mass ratio) under ambient conditions. When gently mixing sulfur and porous carbon, complete surface coverage takes place within just a few days along with the loss of sulfurs bulk properties (crystallinity, melting point, Raman signals). Sulfur spillover also occurs in presence of a liquid phase. Consequences of this phenomenon are discussed using a sodium-sulfur cell with solid electrolyte membrane. Overall, sulfur spillover on carbon is a so far overlooked phenomenon that we expect to have major relevance for all types of metal-sulfur batteries with porous carbon as support.
L. Medenbach, I. Escher, N. Köwitsch, M. Armbrüster, L. Zedler, B. Dietzek, P. Adelhelm
Angew. Chem. Int. Ed. 57, 2018, 13666.
doi: 10.1002/anie.201807295
|
 |
Formic Acid Decomposition over ZnPd — Implications for Methanol Steam Reforming
 Single-phase ZnPd with different elemental composition as well as ZnO-supported ZnPd nanoparticles were prepared and tested concerning their catalytic properties in the formic acid decomposition with the aim to investigate a possible formate pathway in the steam reforming of methanol (MSR). Pd-rich ZnPd showed higher catalytic activity than ZnPd with lower Pd content. All samples showed high and stable CO2 selectivity. The stability of the materials was investigated both ex situ by powder X-ray diffraction (XRD), scanning electron microscopy (SEM), and Raman spectroscopy after formic acid decomposition as well as by in situ X-ray photoelectron spectroscopy (in situ XPS) and operando differential thermal analysis–thermogravimetry (DTA/TG). Samples with higher Pd content exhibited higher stability against oxidation, corroborating earlier observations under MSR conditions. The supported ZnPd/ZnO material and Zn-rich bulk ZnPd samples showed a strong modification after reaction, which is attributed to zinc formate formation during formic acid decomposition. Kinetic data give strong indications to exclude dehydrogenation of a formate intermediate as rate-limiting step in MSR. Single-phase ZnPd with different elemental composition as well as ZnO-supported ZnPd nanoparticles were prepared and tested concerning their catalytic properties in the formic acid decomposition with the aim to investigate a possible formate pathway in the steam reforming of methanol (MSR). Pd-rich ZnPd showed higher catalytic activity than ZnPd with lower Pd content. All samples showed high and stable CO2 selectivity. The stability of the materials was investigated both ex situ by powder X-ray diffraction (XRD), scanning electron microscopy (SEM), and Raman spectroscopy after formic acid decomposition as well as by in situ X-ray photoelectron spectroscopy (in situ XPS) and operando differential thermal analysis–thermogravimetry (DTA/TG). Samples with higher Pd content exhibited higher stability against oxidation, corroborating earlier observations under MSR conditions. The supported ZnPd/ZnO material and Zn-rich bulk ZnPd samples showed a strong modification after reaction, which is attributed to zinc formate formation during formic acid decomposition. Kinetic data give strong indications to exclude dehydrogenation of a formate intermediate as rate-limiting step in MSR.
R. Kriegel, D.C.A. Ivarsson, M. Armbrüster
ChemCatChem 10, 2018, 2664.
doi: 10.1002/cctc.201800194
|
 |
Simple Vapor-Solid Synthesis of Zn-Based Intermetallic Compounds
 A synthesis route to ZnPd, ZnPt and Cu5Zn8 based on a vapor-solid reaction of stoichiometric amounts of the elements is presented. The high vapor pressure of zinc, usually hindering a controlled synthesis, forms part of the developed synthesis strategy with a focus on the catalytically active intermetallic compound ZnPd. The method provides excellent compositional control of better than 0.1 at% and results in dense samples without requiring detailed knowledge of the corresponding thermodynamics. Utilizing the well-defined ZnPd samples, the bulk defect model as well as the reducibility of surface oxides was clarified. The well-controlled synthesis route creates new opportunities for exploring the reaction kinetics and formation processes of intermetallic compounds. A synthesis route to ZnPd, ZnPt and Cu5Zn8 based on a vapor-solid reaction of stoichiometric amounts of the elements is presented. The high vapor pressure of zinc, usually hindering a controlled synthesis, forms part of the developed synthesis strategy with a focus on the catalytically active intermetallic compound ZnPd. The method provides excellent compositional control of better than 0.1 at% and results in dense samples without requiring detailed knowledge of the corresponding thermodynamics. Utilizing the well-defined ZnPd samples, the bulk defect model as well as the reducibility of surface oxides was clarified. The well-controlled synthesis route creates new opportunities for exploring the reaction kinetics and formation processes of intermetallic compounds.
D.C.A. Ivarsson, U. Burkhardt, K. Richter, R. Kriegel, L. Rößner, M. Neumann, M. Armbrüster
J. All. Compds 743, 2018, 155.
doi: 10.1016/j.jallcom.2018.01.385
|
 |
On the twinning in ZnPd
 The intermetallic compound ZnPd has demonstrated excellent catalytic properties in methanol steam reforming. While it is known that defects and microstructures influence the catalytic properties, little is known about the defects occurring in ZnPd. Due to recent advances in synthetic methods, coarse-grained ZnPd samples are accessible. This enables the detection and investigation of twinning in ZnPd by studying the twinned regions from the macroscopic scale by polarised light and electron backscattering diffraction (EBSD) down to the atomic scale by high-resolution transmission electron microscopy (HR-TEM). Twinning occurs in {101} and is coupled with a change in the c/a ratio in the vicinity of the twin boundary. Quantum chemical calculations result in only very small energy differences between the ideal and the twinned structure, explaining the experimentally observed thermal stability of the latter. The chemical bonding was investigated by the electron localizability indicator (ELI) and compared to the one in the ideal structure. The results confirm twinning along the {101} plane and demonstrate the high stability of the twin boundaries after formation. The intermetallic compound ZnPd has demonstrated excellent catalytic properties in methanol steam reforming. While it is known that defects and microstructures influence the catalytic properties, little is known about the defects occurring in ZnPd. Due to recent advances in synthetic methods, coarse-grained ZnPd samples are accessible. This enables the detection and investigation of twinning in ZnPd by studying the twinned regions from the macroscopic scale by polarised light and electron backscattering diffraction (EBSD) down to the atomic scale by high-resolution transmission electron microscopy (HR-TEM). Twinning occurs in {101} and is coupled with a change in the c/a ratio in the vicinity of the twin boundary. Quantum chemical calculations result in only very small energy differences between the ideal and the twinned structure, explaining the experimentally observed thermal stability of the latter. The chemical bonding was investigated by the electron localizability indicator (ELI) and compared to the one in the ideal structure. The results confirm twinning along the {101} plane and demonstrate the high stability of the twin boundaries after formation.
D.C.A. Ivarsson, U. Burkhardt, M. Heggen, A. Ormeci, M. Armbrüster
PhysChemChemPhys 19, 2017, 5778.
doi: 10.1039/C6CP08117G
This article is part of themed collection: 2017 PCCP HOT Articles
|
 |
ZnPd/ZnO Aerogels as Potential Catalytic Materials
 Aerogels are interesting materials, consisting out of air and therefore can have high surface areas. This work aimed to use this property in trying out ZnO-Aerogel as support material for Pd. After a reductive step Pd should react with the support material to form the intermetallic compound ZnPd. In the picture you see the successful result of this work in the example reaction of methanol steam reforming, showing that ZnPd/ZnO-aerogels are more active than conventional ZnPd/ZnO, while still maintaining the high CO2-selectivity of ZnPd. Aerogels are interesting materials, consisting out of air and therefore can have high surface areas. This work aimed to use this property in trying out ZnO-Aerogel as support material for Pd. After a reductive step Pd should react with the support material to form the intermetallic compound ZnPd. In the picture you see the successful result of this work in the example reaction of methanol steam reforming, showing that ZnPd/ZnO-aerogels are more active than conventional ZnPd/ZnO, while still maintaining the high CO2-selectivity of ZnPd.
C. Ziegler, S. Klosz , L. Borchardt, M. Oschatz, S. Kaskel, M. Friedrich, R. Kriegel, T. Keilhauer, M. Armbrüster, A. Eychmüller
Adv. Func. Mater. 26, 2015, 1014.
doi: 10.1002/adfm.201503000
|
 |
Travel stipend for ICC 2016 granted
 For the upcoming 16th International Congress on Catalysis (ICC), which will take place July 3rd to 8th 2016 in Beijing, China, a travel stipend was granted to our PhD student René Zimmermann. The German Catalysis Society (GeCatS) is thankfully acknowledged for granting one of in total five stipends to the professorship of Materials for Innovative Energy Concepts. For the upcoming 16th International Congress on Catalysis (ICC), which will take place July 3rd to 8th 2016 in Beijing, China, a travel stipend was granted to our PhD student René Zimmermann. The German Catalysis Society (GeCatS) is thankfully acknowledged for granting one of in total five stipends to the professorship of Materials for Innovative Energy Concepts.
|
 |
Solid/Gas-Reaction Obtaining Supported Intermetallic Ga-Pd Catalyst Materials
 The picture shows the transformation of a commercially available supported palladium catalyst (Pd/C) into supported particles of the intermetallic compound GaPd2. GaPd2 as well as GaPd can be obtained by controlled solid-gas phase reaction, opening a new path to the synthesis of finely dispersed, small and supported intermetallic materials. The basis for the optimized synthesis is formed by thermodynamic data, i.e. the phase diagram, as well as chemical vapor deposition experiments, by which large crystals of GaPd2 can be obtained. The supported materials show very promising catalytic properties, e.g. in the semi-hydrogenation of acetylene. Synthesis of supported materials allows to obtain high-performance materials which combine the excellent catalytic selectivity of unsupported bulk intermetallic compounds with high activity, thus easing industrial application. The picture shows the transformation of a commercially available supported palladium catalyst (Pd/C) into supported particles of the intermetallic compound GaPd2. GaPd2 as well as GaPd can be obtained by controlled solid-gas phase reaction, opening a new path to the synthesis of finely dispersed, small and supported intermetallic materials. The basis for the optimized synthesis is formed by thermodynamic data, i.e. the phase diagram, as well as chemical vapor deposition experiments, by which large crystals of GaPd2 can be obtained. The supported materials show very promising catalytic properties, e.g. in the semi-hydrogenation of acetylene. Synthesis of supported materials allows to obtain high-performance materials which combine the excellent catalytic selectivity of unsupported bulk intermetallic compounds with high activity, thus easing industrial application.
M. Schmidt, K. Kovnir, J. Deichsel, M. Binnewies, Y. Grin, M. Armbrüster
Z. Anorg. Allg. Chem. 641, 2015, 1061.
doi: 10.1002/zaac.201500095
|
 |
Reactive Metal-Support Interaction (RMSI)
 Oxide supported metal particles are often used as heterogeneous catalysts in a large number of industrial processes. Under reaction conditions – or during conditioning of these materials – the metallic species can react with the oxidic support to form substitutional alloys or structurally well-ordered intermetallic compounds. This results in a strong alteration of the catalytic properties due to the change of the electronic and structural properties of the metallic phase. Reviewing the literature reveals how common this very important process is in catalysis. Oxide supported metal particles are often used as heterogeneous catalysts in a large number of industrial processes. Under reaction conditions – or during conditioning of these materials – the metallic species can react with the oxidic support to form substitutional alloys or structurally well-ordered intermetallic compounds. This results in a strong alteration of the catalytic properties due to the change of the electronic and structural properties of the metallic phase. Reviewing the literature reveals how common this very important process is in catalysis.
S. Penner, M. Armbrüster
ChemCatChem, 7, 2015, 374.
doi: 10.1002/cctc.201402635
|
 |
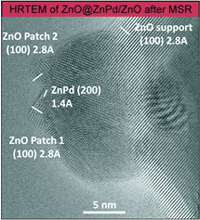
Metal’s Little Helper: Zinc Oxide Enables Clean Hydrogen Production From Methanol
 Methanol steam reforming (MSR, CH3OH + H2O → 3H2 + CO2) is a promising way to release hydrogen from methanol and water for mobile fuel cell application. The focus however is on the suppression of CO formation as CO poisons fuel cell catalysts. Methanol steam reforming (MSR, CH3OH + H2O → 3H2 + CO2) is a promising way to release hydrogen from methanol and water for mobile fuel cell application. The focus however is on the suppression of CO formation as CO poisons fuel cell catalysts.
Until now, the low CO formation monitored on well-studied ZnPd/ZnO catalysts was solely attributed to the existence of intermetallic ZnPd on the ZnO support.
Very recently, experiments using aberration-corrected, high-resolution TEM and STEM proved the dynamic nature of the catalyst, forming small patches of zinc oxide on the intermetallic ZnPd nanoparticles, as soon as MSR conditions are applied. The high CO2 selectivity of the catalyst thus has to be assigned to the synergistic effect of ZnPd and ZnO.
M. Friedrich, S. Penner, M. Heggen, M. Armbrüster
Angew. Chem. Int. Ed. 52, 2013, 4389.
doi: 10.1002/anie.201209587
|
 |













 A sustainable energy infrastructure requires chemical energy conversion, i.e. the storage of renewable energy in small chemical molecules and the release of the stored energy upon demand. Electrocatalysis, comprising reactions like hydrogen generation by water splitting, takes a central role in implementing many of the necessary reactions. With their ordered structure, intermetallic compounds provide a unique combination of crystal and electronic structure, thus new (electro)chemical properties. This review comprises nearly 350 publications since 1970 using intermetallic compounds as electrocatalysts in energy-relevant reactions. Special emphasis lies on the so-far applied optimization strategies involving the peculiar properties of the class of intermetallic compounds. Understanding the versatility of intermetallic compounds opens possibilities for further development of heterogeneous catalysts, e.g. electrodes for electrochemical conversions.
A sustainable energy infrastructure requires chemical energy conversion, i.e. the storage of renewable energy in small chemical molecules and the release of the stored energy upon demand. Electrocatalysis, comprising reactions like hydrogen generation by water splitting, takes a central role in implementing many of the necessary reactions. With their ordered structure, intermetallic compounds provide a unique combination of crystal and electronic structure, thus new (electro)chemical properties. This review comprises nearly 350 publications since 1970 using intermetallic compounds as electrocatalysts in energy-relevant reactions. Special emphasis lies on the so-far applied optimization strategies involving the peculiar properties of the class of intermetallic compounds. Understanding the versatility of intermetallic compounds opens possibilities for further development of heterogeneous catalysts, e.g. electrodes for electrochemical conversions. Oxide supported metal particles are often used as heterogeneous catalysts in a large number of industrial processes. Under reaction conditions – or during conditioning of these materials – the metallic species can react with the oxidic support to form substitutional alloys or structurally well-ordered intermetallic compounds. This results in a strong alteration of the catalytic properties due to the change of the electronic and structural properties of the metallic phase. Reviewing the literature reveals how common this very important process is in catalysis.
Oxide supported metal particles are often used as heterogeneous catalysts in a large number of industrial processes. Under reaction conditions – or during conditioning of these materials – the metallic species can react with the oxidic support to form substitutional alloys or structurally well-ordered intermetallic compounds. This results in a strong alteration of the catalytic properties due to the change of the electronic and structural properties of the metallic phase. Reviewing the literature reveals how common this very important process is in catalysis. Methanol steam reforming (MSR, CH3OH + H2O → 3H2 + CO2) is a promising way to release hydrogen from methanol and water for mobile fuel cell application. The focus however is on the suppression of CO formation as CO poisons fuel cell catalysts.
Methanol steam reforming (MSR, CH3OH + H2O → 3H2 + CO2) is a promising way to release hydrogen from methanol and water for mobile fuel cell application. The focus however is on the suppression of CO formation as CO poisons fuel cell catalysts.